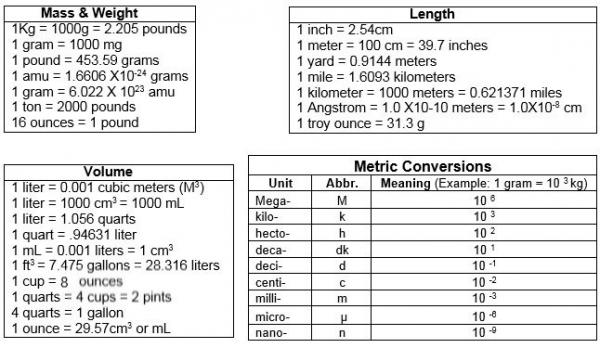
- Avogadro's number Avogadro's number is the number of particles in one mole of any substance. Its numerical value is 6.02225 × 1023. One mole of oxygen gas contains 6.02 × 1023molecules of oxygen, while one mole of sodium chloride contains 6.02 × 1023sodium ions and 6.02 × 1023 chloride ions.
- Avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. The units may be electrons, atoms, ions, or molecules, depending on the nature of the substance and the character of the reaction (if any).See alsoAvogadro’s law.
How do we use mathematical shorthand to write very large and very small numbers? We focus on the most important digits, and use an abbreviation to avoid writing out the insignificant digits.
Avogadro's Number In Scientific Notation
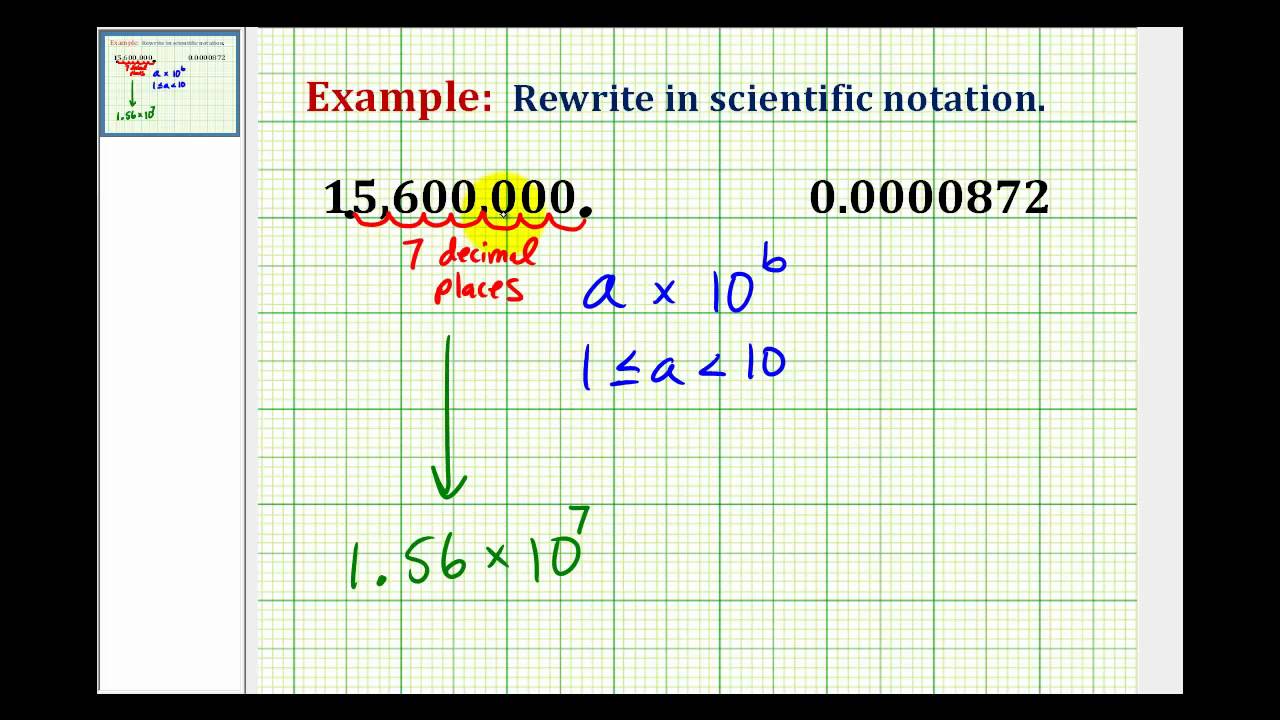
Large numbers can be written in scientific notation by moving the decimal point to the left. For example, Avogadro's number is approximately 602,200,000,000,000,000,000,000 (where the decimal point implicitly resides to the right of the last zero). Using scientific notation this impressive number is simplified to: 6.022 x 10 23.
Usually the only significant information is in the first couple of digits and the location in place value that they occupy.
Scientific Notation
- Allows us to compactly write very large or very small numbers
- Creates a shorthand that allows us to simplify calculation and avoid errors
- Note that you already use scientific notation when you speak about large numbers
- You don’t say one zero zero zero zero zero zero
- You say one million
Orders of magnitude
- Powers of ten
- Place value representation
- Operating on numbers with scientific notation
Representing large numbers

There are different ways to express scientific notation

- On paper 1.23 times 10^4
- On a calculator 1.23 (EE key) 4
- On a computer
1.23E4
A very large number
- Avogadro’s Number
- 6.02 times 10^{23}
- 10^{1} = 10
- 10^{2} = 10 times 10
- 10^{3} = 10 times 10 times 10
A very small number
- Gravitational Constant
- 6.67 times 10^{-11} (m^3 kg^{-1} s^{-2})
- 10^{-1} = frac{1}{10}
- 10^{-2} = frac{1}{10 times 10}
- 10^{-3} = frac{1}{10 times 10 times 10}
Avogadro's Number In Scientific Notation Calculator
Operations
- Addition
- Subtraction
- Multiplication
- Division
Multiplying and dividing large numbers
10^a cdot 10^b = 10^{a+b}
c cdot 10^a cdot d cdot 10^b = c cdot d cdot 10^{a+b}
frac{10^a}{10^b} = 10^{a-b}
frac{c cdot 10^a}{d cdot 10^b} = frac{c}{d} cdot 10^{a-b}
You can combine these rules if you are multiplying and dividing several numbers in scientific notation.
Common Powers
Notice that these prefixes correspond to english language quantities you are already familiar with. You often express large numbers verbally, so there isn’t much new to learn to use metric prefixes or scientific notation.
For example, 1 billion joules is the same as a gigajoule is the same as 1 cdot 10^{9} joules is the same as 1,000,000,000 joules.
A more complete list can be found here: Wikipedia Metric Prefixes
| greek prefix | english name | exponent |
|---|---|---|
| kilo | thousand | 10^3 |
| mega | million | 10^6 |
| giga | billion | 10^9 |
| tera | trillion | 10^{12} |
| peta | quadrillion | 10^{15} |
| exa | quintillion | 10^{18} |
